Bioheng's CD7 UCAR-T and Allogeneic HSCT Sequential Therapy Study Published in the New England Journal of Medicine

On April 25, 2024, the New England Journal of Medicine published the latest findings by the team of He Huang, M.D., PhD., and Yongxian Hu, M.D., PhD., from the First Affiliated Hospital, Zhejiang University School of Medicine, titled "Sequential CD7 CAR-T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis"[1]. The study introduces an innovative treatment strategy that combines CD7 CAR-T with allogeneic hematopoietic stem cell transplantation (allo-HSCT) for the first time.
Professors He Huang, Yongxian Hu, and Dongrui Wang from the Hematopoietic Stem Cell Transplantation Center at the First Affiliated Hospital of Zhejiang University School of Medicine, along with Professor Hongsheng Zhang from the School of Life Sciences at Fudan University, are the co-corresponding authors of this paper. Bioheng participated in the trial, provided trial drug CD7 UCAR T, and Ren Jiangtao, PhD., Chief Scientist of Bioheng Therapeutics participated in the authorship.
"Integrated" Strategy
Patients with relapsed or refractory hematological tumors have a poor prognosis, with an overall 5-year survival rate of less than 20% [2][3][4]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a key strategy for treating such diseases but faces limitations due to complications such as graft-versus-host disease (GVHD), toxicity related to conditioning regimen, and severe immunosuppression following long-term anti-GVHD treatment, making it difficult to apply to patients with relatively poor general conditions[5][6].
The team led by Professor Huang He from the First Affiliated Hospital of Zhejiang University School of Medicine has been committed to the research and clinical translation of CD7 CAR-T for the treatment of T-cell malignant hematological tumors[7], they have developed a new "integrated" treatment strategy. This strategy involves sequential allogeneic CD7 CAR-T followed by hematopoietic stem cell transplantation (Figure 1).
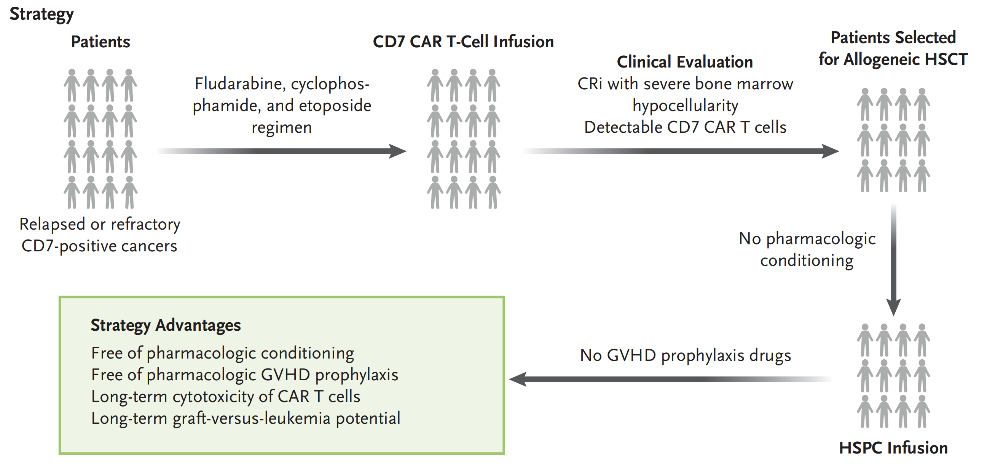
Figure 1: Schematic of the "Integrated" Treatment Strategy
Patients receive CD7 chimeric antigen receptor (CAR) T-cell therapy and achieve complete remission (CR) with incomplete hematological recovery (CRi), followed by non-myeloablative haploidentical hematopoietic stem cell transplantation (HSCT) without GVHD prophylaxis. This innovative strategy allows for the preservation of CAR-T cells, full donor chimerism, and prolongs the leukemia-free period.
HSPC: Hematopoietic stem cells and progenitor cells.
The "integrated" strategy innovatively leverages the bone marrow suppression state following CD7 CAR-T cell therapy in patients, achieving the effects comparable to high-dose myeloablative pre-transplant chemotherapy. It also leverages the characteristic of CAR-T cells to eliminate CD7-positive normal T lymphocytes, which to a certain extent prevents GVHD caused by newly generated CD7-positive T cells. At the same time, the sustained persistence of CAR-T cells, together with the graft-versus-leukemia (GVL) effect, jointly contributes to the prevention of relapse.
Outcomes and safety
In terms of efficacy, among the 10 patients treated, six remained in minimal residual disease (MRD) negative complete remission at a median follow-up of 15.1 months. The estimated one-year overall survival (OS) rate is 68% (95% CI, 43-100), and the one-year disease-free survival (DFS) rate is 54% (95% CI, 29-100) (Figure 2).
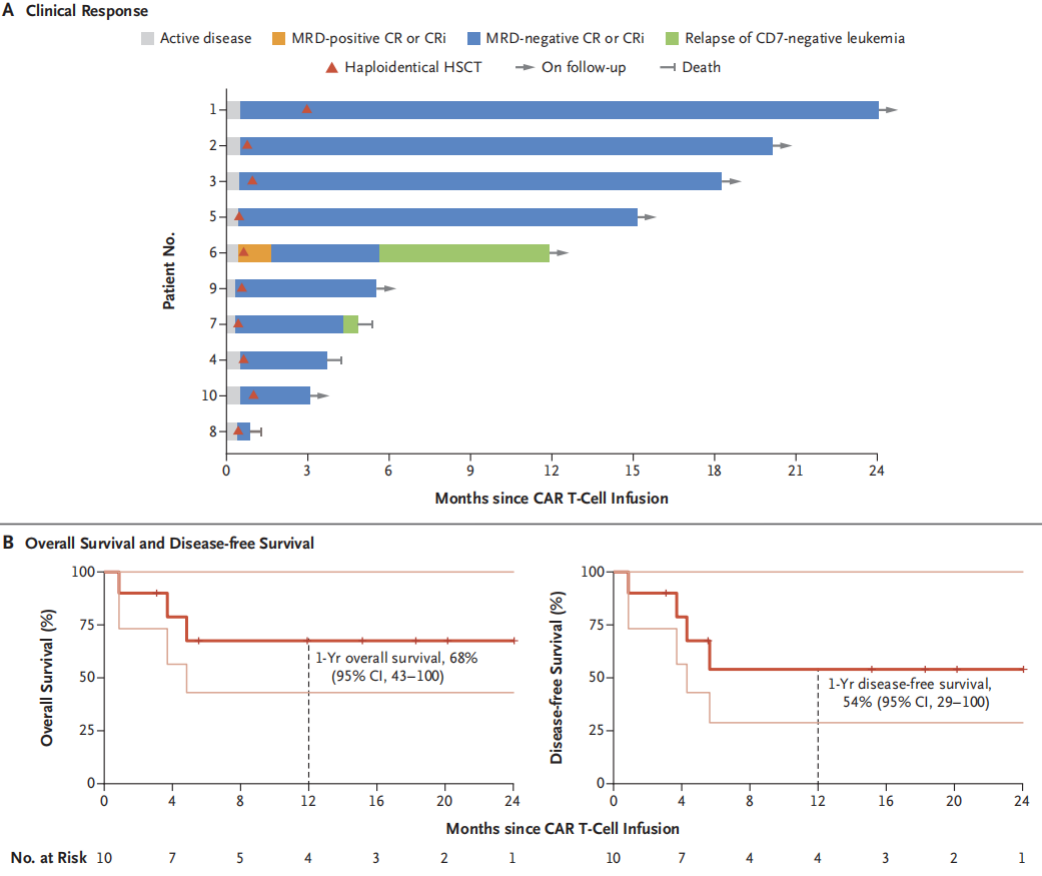
Figure 2: Efficacy and Long-term Survival
Regarding GVHD control, without the use of GVHD prophylaxis drugs, only three patients experienced low-grade acute GVHD, and none developed chronic GVHD.
Following CAR-T therapy, significant in vivo expansion was observed in all patients. By the cutoff date, CAR-T cells were still detectable in five of the patients with donor engraftment (Figure 3).
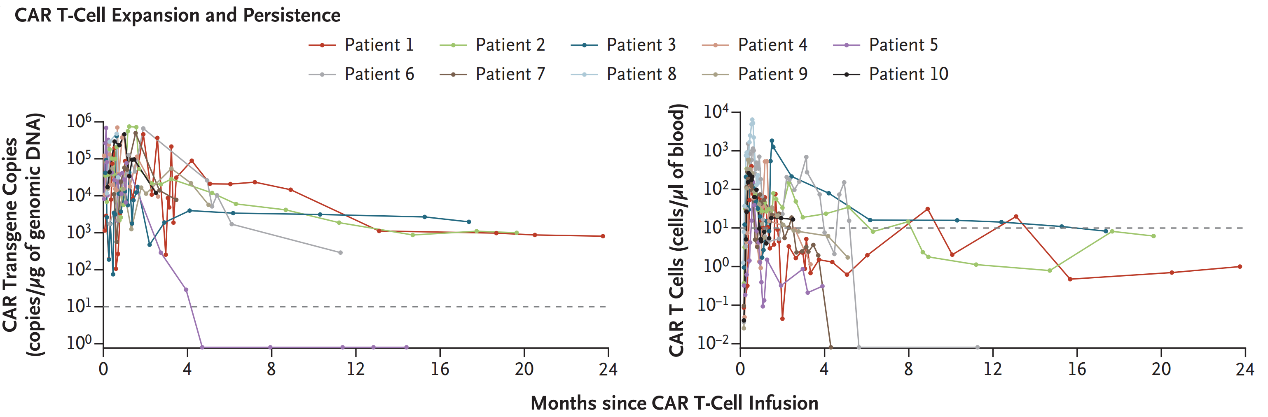
Figure 3: CAR-T Amplification and Persistence
About Bioheng
Bioheng Therapeutics is a clinical-stage company focused on allogeneic " off-the-shelf " universal CAR-T therapies,aiming to develop the world’s leading allogeneic cell therapy platforms and products to address some of the most challenging unmet needs.
In June 2021, its CD7-targeted UCAR-T, received Orphan Drug Designation (ODD) from the U.S. FDA for the treatment of r/r T-ALL/LBL. This product is based on Bioheng's novel and proprietary UCAR-T technology platform - ANSWER®. Bioheng is progressing the global clinical trial for this pipeline. Built upon ANSWER® platform, Bioheng is also actively developing multiple UCAR-T pipelines.
References:
- Hu Y, Zhang M, Yang T, Mo Z, Wei G, Jing R, Zhao H, Chen R, Zu C, Gu T, Xiao P, Hong R, Feng J, Fu S, Kong D, Xu H, Cui J, Huang S, Liang B, Yuan X, Cui Q, Guo H, Yu Y, Feng Y, Jin C, Ren J, Chang AH, Wang D, Huang H. Sequential CD7 CAR-T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis. N Engl J Med. 2024 Apr 25;390(16):1467-1480.
- Ganzel C, Sun Z, Cripe LD, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: the ECOG-ACRIN experience. Am J Hematol 2018; 93: 1074-81.
- Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet 2020; 395: 1146-62.
- Penack O, Peczynski C, Mohty M, et al. Association of pre-existing comorbidities with outcome of allogeneic hematopoietic cell transplantation: a retrospective analysis from the EBMT. Bone Marrow Transplant 2022; 57: 183-90.
- Zhao H, Wei J, Wei G, et al. Pre-transplant MRD negativity predicts favorable outcomes of CAR-T therapy followed by haploidentical HSCT for relapsed/refractory acute lymphoblastic leukemia: a multicenter retrospective study. J Hematol Oncol 2020; 13: 42.
- Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, Cui Q, Mu Q, Chen G, Han L, Guo T, Cui J, Jiang X, Zheng X, Yu S, Li X, Zhang X, Chen M, Li X, Gao M, Wang K, Zu C, Zhang H, He X, Wang Y, Wang D, Ren J, Huang H. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022 Nov;32(11):995-1007.
Related News
Contact Us







