EHA 2025 | Bioheng Therapeutics Presents Latest Clinical Data on Universal CAR-T Therapy CTD402 for Patients with R/R T-ALL/LBL
The report at this EHA Congress detailed the results of a multi-center, investigator-initiated Phase I/II pooled analysis of CTD402 for the treatment of R/R T-ALL/LBL. Between December 2021 and November 2023, a total of 62 patients with R/R T-ALL/LBL (including 46 with ALL and 16 with LBL) were enrolled and received treatment. The median age of the patients was 23 years, and they had received a median of 3 prior lines of therapy. The median baseline blast percentage in the bone marrow of the patients was 45.1%, and 58.1% (36 out of 62) of the patients presented with extramedullary disease (EMD).
Safety
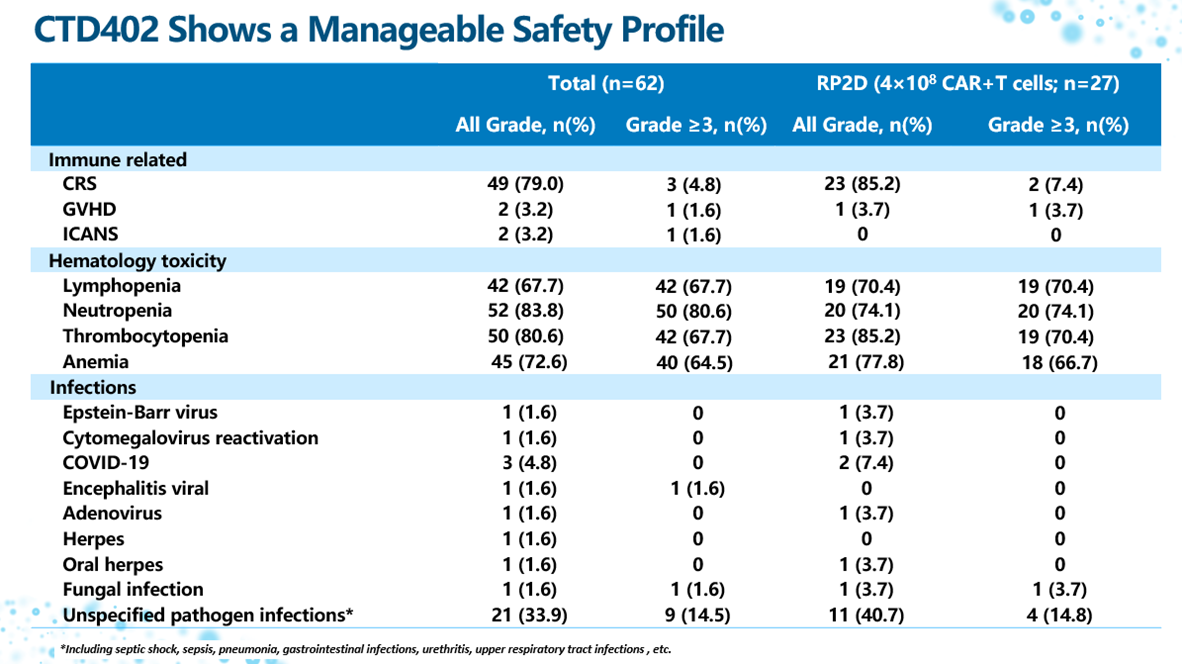
CTD402 demonstrated a favorable safety profile in this study:
- Cytokine Release Syndrome (CRS): The overall incidence was 79.0%, but cases were predominantly low-grade. The incidence of Grade 3 or higher CRS was only 4.8% (3/62).
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS): Only 2 cases were observed.
- Graft-versus-Host Disease (GVHD): Only 2 cases were observed.
- Other Adverse Events (AEs): The most common Grade 3 or higher AEs were hematologic toxicities. The incidence of infection events was low.
Efficacy
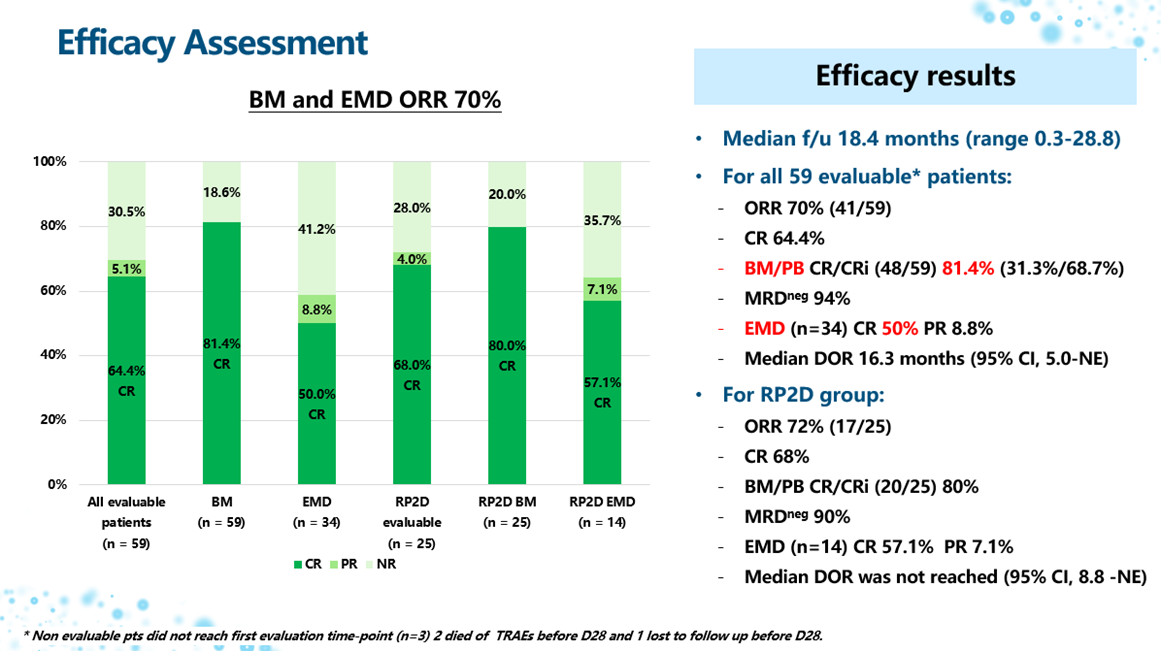
As of March 13, 2025, the median follow-up time was 18.4 months.
In the 59 evaluable patients overall, the efficacy outcomes were positive:
- Response Rate: The complete remission (CR) rate was 64.4% (38/59), and the bone marrow/peripheral blood remission rate was 81.4% (48/59). For the 34 patients with baseline extramedullary disease (EMD), the extramedullary remission rate was 50% (17/34).
- Depth and Duration of Remission: Among patients who achieved CR, 94.7% (36/38) achieved minimal residual disease (MRD) negativity. The median duration of remission (DOR) was 16.3 months (95% CI, 5.0-NE).
In the Recommended Phase 2 Dose (RP2D, 4×108 CAR-T cells, n=25) cohort, superior efficacy was observed:
- In this group, the CR rate reached 68.0% (17/25), the bone marrow/peripheral blood remission rate was 80.0% (20/25), and the EMD remission rate was 57.1% (8/14).
- Notably, the median DOR for this dose group has not yet been reached (95% CI, 8.8-NE), indicating potential for durable remission.
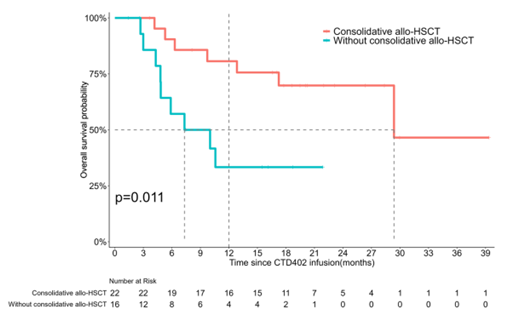
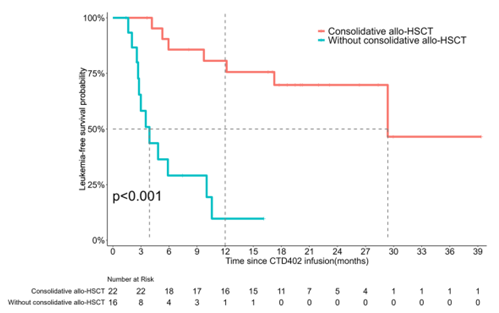
Long-term Benefit: Consolidative Transplantation Significantly Extends Survival
For patients who achieve remission, the subsequent treatment strategy is crucial. The study found that among the 38 patients who achieved a complete remission (CR), 22 (58%) received consolidative hematopoietic stem cell transplantation (HSCT). Compared to patients who did not undergo transplantation, the median overall survival (OS) and median leukemia-free survival (LFS) of the transplant group were both significantly prolonged (OS: 29.4 months vs. 8.7 months, p=0.011; LFS: 29.4 months vs. 3.9 months, p<0.001), indicating that consolidative transplantation provides long-term survival benefits for these patients.
For more information about the oral presentation and the EHA Annual Congress, please visit the official EHA website.
https://congress-apps.ehaweb.org/eha2025/en-GB/pag/presentation/566395
About CTD402
CTD402 is an allogeneic, CD7-targeting Universal CAR (UCAR) T-cell therapy product derived from healthy donors for the treatment of T-ALL/LBL and other T-cell hematological malignancies. The product is gene-edited to prevent fratricide, graft-versus-host disease (GvHD), and host-versus-graft (HvG) rejection, while simultaneously enhancing its anti-tumor activity. CTD402 can be manufactured from a single batch to treat multiple patients, providing an "off-the-shelf" solution for patients in need of CAR-T cell therapy.
Related News
Contact Us







