Pipeline
Our pipelines focus on oncology and autoimmune diseases

Technology Platforms
Our platforms – featuring ANSWER® CAR, Explored CAR® and RVVpacReady®
ANSWER® 2nd Gen UCAR-T
Onco & Non-onco
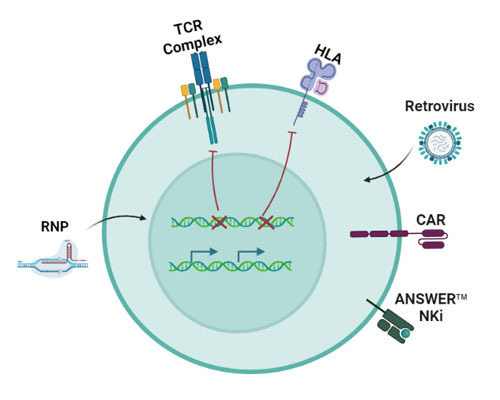
NK inhibition strategy: KO TCR, HLA and overexpress NK inhibitory molecule
Explored CAR®
Solid Tumor
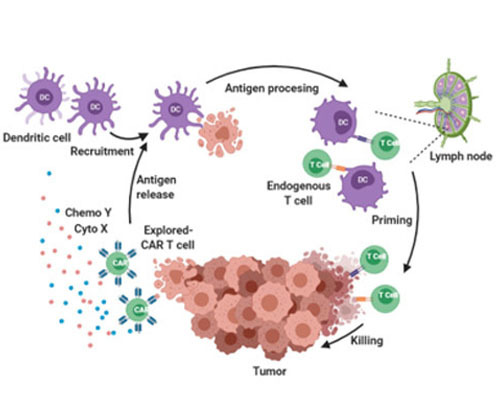
Solid tumor Armored CAR-T strategy: Secrete payload cytokine and chemokine to evoke in situ immune response
RVVpacReady®
Viral Vector
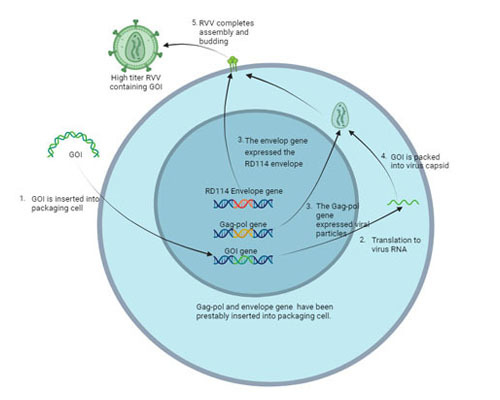
Higher harvest titer and transduction efficiency with lower COGS and impurities
Publications
Recently published articles
Background: T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) are highly aggressive T-lineage malignancies characterized by an infiltration of immature T cells in the bone marrow (BM) and peripheral blood (PB) or extramedullary organ involvements. Effective treatments were elusive, and the long-term survival of patients with refractory/relapsed (R/R) T-ALL/LBL remains poor despite allogeneic hematopoietic stem cell transplantation (allo-HSCT). CAR-T therapies for T-cell malignancies remain underdeveloped due to the shared antigenicity between normal and malignant T cells. CD7 is highly expressed on the surface of T-ALL/LBL T cells and is considered a viable CAR-T therapeutic target. However, CD7-targeting immunotherapies may be compromised by T-cell fratricide and extermination. And one of the biggest challenges with autologous CAR-T cell therapy is to isolate a sufficient number of healthy T cells from malignant T cells. Thus, clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR9-associated protein 9 (Cas9)-mediated gene ablation has been used to produce universal CD7-targeted CAR T cells (UCD7-CAR) from suitable healthy donors that no longer express CD7 and TCR. In vitro killing tests, animal tests, and phase 1 clinical trials have preliminarily verified the efficacy and safety of universal CD7 CAR-T cells. Here we investigated the safety and efficacy of the universal CD7 CAR-T therapy in phase 2 clinical trial(NCT05454241) for pediatric R/R T-ALL/LBL patients.
Although autologous CAR-T therapy targeting CD19+ B-cell has proven efficacy in hematological malignancies. The challenges such as undesirable waiting time, possible manufacturing failures and high cost, remain to be solved. In addition, antigen-loss/downregulation leads to treatment failure after single-target CAR-T therapy. To tackle these issues, we developed a universal CD19/CD22-targeting CAR-T cell with the TRAC region and CD52 gene disrupted using CRISPR/Cas9 technology. Pre-clinical data demonstrated the safety of CRISPR gene editing and the anti-leukemia ability of CTA101.
Contact Us






