EHA2024 | Bioheng Therapeutics Announces Latest Clinical Data of CD7-targeted Universal CAR-T Cell Product RD13-02 for Relapsed/Refractory T-cell Acute Lymphoblastic Leukemia/Lymphoma (R/R T-ALL/LBL) Patients, with an Impressive Response Rate of Up to 92%
On May 16, 2024, Bioheng Therapeutics (referred to as "Bioheng"), an innovative biopharmaceutical company focusing on the development and commercialization of Universal Chimeric Antigen Receptor T-cell (UCAR-T) products, announced today that it will present the Phase I clinical data of its CD7-targeted Universal CAR-T cell product, RD13-02, for the treatment of relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoma (R/R T-ALL/LBL) patients at the 29th European Hematology Association (EHA 2024) Annual Congress, to be held in Madrid, Spain, from June 13 to 16, 2024, in the form of an academic poster.
Abstract Title: Phase I Study of an Anti-CD7 Universal CAR-T Therapy for Patients with Relapsed/Refractory CD7 Positive T-ALL/LBL
Abstract Number: P1471
The data presented at this EHA conference pertains to an investigator-initiated Phase I clinical trial of RD13-02 conducted at the First Affiliated Hospital of Zhejiang University School of Medicine. This single-arm, open-label, dose-escalation Phase I study (NCT05716113) evaluated the safety and efficacy of RD13-02 in treating patients with R/R T-ALL/LBL. The study consisted of two stages: dose escalation (using an accelerated titration design) and dose expansion, with a total of 4 dose levels (DL-1: 0.5×108 CAR+T cells; DL1: 2×108 CAR+T cells; DL2: 4×108 CAR+T cells; DL3: 6×108 CAR+T cells). All patients received fludarabine (30 mg/m2/day for 3 days) and cyclophosphamide (500 mg/m2/day for 3 days) conditioning regimen prior to RD13-02 infusion.
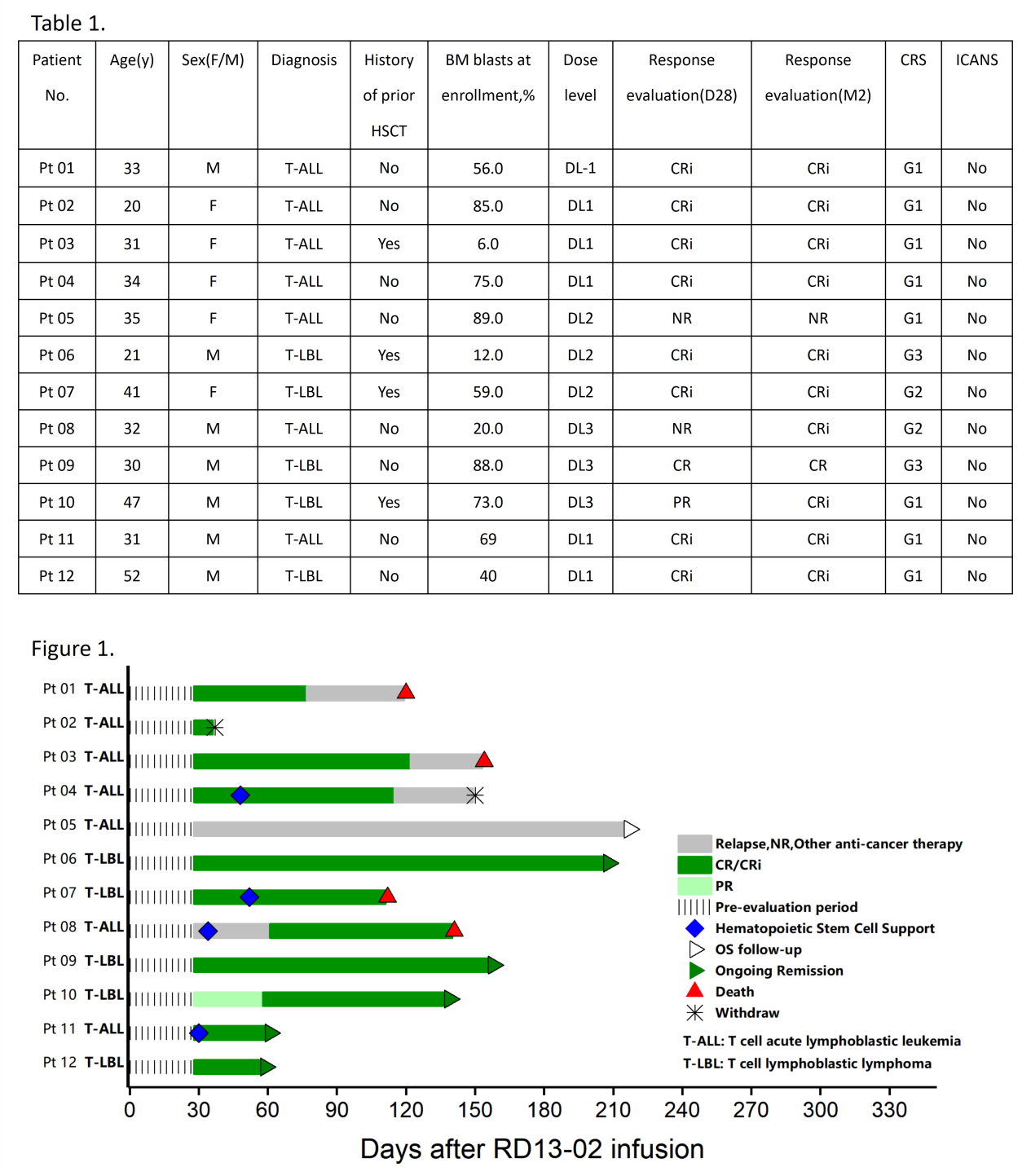
As of February 21, 2024, a total of 12 eligible patients with R/R T-ALL/LBL have been enrolled (7 ALL, 5 LBL), with a median age of 32.5 years (range, 20 to 52). The median prior lines of therapy were 2 (range, 1 to 4), and 4 patients had prior HSCT before enrollment. The median proportion of BM blasts was 64% (range: 6% to 89%) among all 12 patients, with 6 (3 ALL, 3 LBL) showing extramedullary involvement.
Safety:
No dose-limiting toxicity (DLT) event, neurotoxicity and GvHD have been observed at all dose levels. Cytokine release syndrome (CRS) was manageable with majority being mild (G1, n=8; G2, n=2; G3, n=2). Due to the occurrence of Grade 3 CRS in one patient each from DL2 and DL3 groups, investigators set the DL1 as expansion dose with a paramount focus on safety and efficacy.
Efficacy:
At the initial 28-day assessment post-infusion, 9 out of 12 patients achieved CR/CRi. While by the 2nd month post-infusion, two more patients reached CR/CRi, with an ORR of 92% (Figure 1). qPCR detected expansion in all patients at the third-party lab, with the exception of Pt11, an HBsAg-positive case, whose expansion was confirmed at the clinical center lab. Time to expansion was between D6 to D15. Peak expansion occurred between D8 to D27. The median duration of persistence was 33 days (15-79).
Conclusion:
The anti-CD7 universal CAR-T product RD13-02 is safe and effective for CD7+ R/R T-ALL/LBL. More patients and longer follow-ups are needed for validation.
About RD13-02
RD13-02 is a universal CAR-T cell product targeting CD7 derived from healthy donors, and intended for the treatment of relapsed or refractory T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL). It is genetically modified to avoid fratricide, graft-versus-host disease (GvHD), and host-versus-graft rejection (HvG) while enhancing anti-tumor activity. RD13-02 can be prepared in a single batch for multiple people, achieving an "off-the-shelf" capability for patients in need of CAR-T cell therapy.
Related News
Contact Us







