ASCO 2025 | Bioheng Therapeutics Presents Positive Clinical Data of the Universal CAR-T Product RD06-03 in R/R B-ALL
June 1, 2025, Bioheng Therapeutics, an innovative biopharmaceutical company focused on the development and commercialization of cell therapies, presented results from an investigator-initiated trial (IIT) of RD06-03, its allogeneic anti-CD19 CAR-T therapy for relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), in a poster session at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting.
Poster Title: A Phase l Study of Allogeneic Anti-CD19 CAR-T Therapy for Patients with CD19+ Relapsed/Refractory Acute B-Lymphobastic Leukemia
Poster Number: 6525
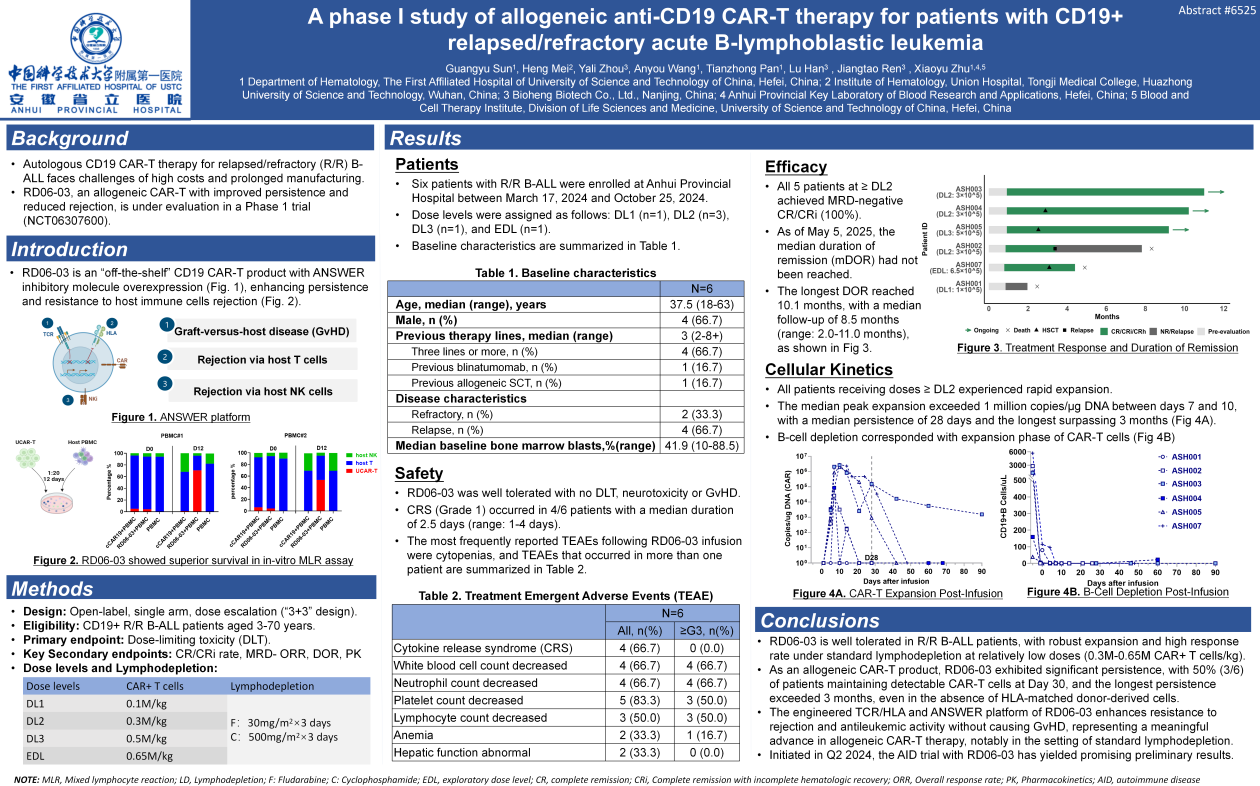
The data presented at ASCO 2025 come from an investigator-initiated Phase I clinical trial conducted at The First Affiliated Hospital of the University of Science and Technology of China. This open-label, single-arm,
dose-escalation trial (NCT06307600) is designed to evaluate the safety and efficacy of RD06-03 in patients with R/R B-ALL. Four dose levels were explored: DL1 (0.1×10⁶ CAR+ T cells/kg), DL2 (0.3×10⁶ CAR+ T cells/kg), DL3 (0.5×10⁶ CAR+ T cells/kg), and EDL (0.65×10⁶ CAR+ T cells/kg). All patients received a standard lymphodepletion regimen of fludarabine (30 mg/m²/day for 3 days) and cyclophosphamide (500 mg/m²/day for 3 days) prior to RD06-03 infusion.
Between March 17, 2024, and October 25, 2024, a total of six patients with R/R B-ALL were enrolled. The median age was 37.5 years (range: 18–63), and patients had received a median of three prior lines of therapy (range: 2 to 8+). One patient had undergone allogeneic hematopoietic stem cell transplantation prior to enrollment. The median baseline bone marrow blast percentage was 41.9% (range: 10%–88.5%).
Safety
No DLT or neurotoxicity were observed at any dose level. CRS occurred in four patients, all of which were mild (Grade 1, n=4), with a median duration of 2.5 days (range: 1–4 days). The most common treatment-emergent adverse events were cytopenias, which were manageable. No severe infection-related adverse events were reported.
Efficacy and Cellular Kinetics
Among the five evaluable patients who received RD06-03 at DL2 or higher, the best ORR was 100% (5/5), with all responders being MRD negative. As of May 5, 2025, the median DOR had not yet been reached. The longest observed remission duration was 10.1 months, with a median follow-up of 8.5 months (range: 2.0–11.0 months). CAR-T cell expansion was detected by qPCR in all patients treated at DL2 or higher, with a median Cmax exceeding one million copies/μg gDNA. The median duration of persistence was 28 days, with the longest persistence observed to exceed 3 months.
Overall, RD06-03 was well tolerated in R/R B-ALL patients, with robust expansion and high response rate at relatively low doses (0.3M-0.65M CAR+ T cells/kg) following standard lymphodepletion . As an allogeneic CAR-T product, RD06-03 exhibited significant persistence, with 50% (3/6) of patients maintaining detectable CAR-T cells at Day 30, and the longest persistence exceeded 3 months, even in the absence of HLA-matched donor-derived cells. The engineered TCR/HLA and ANSWER® platform of RD06-03 enhances its resistance to rejection and antileukemic activity without causing graft-versus-host disease, representing a meaningful advance in allogeneic CAR-T therapy, notably in the setting of standard lymphodepletion.
In Q2 2024, Bioheng Therapeutics initiated exploratory studies of RD06-03 in autoimmune diseases, with preliminary results demonstrating encouraging outcomes.
About RD06-03
RD06-03 is a universal CAR-T cell product targeting CD19 derived from healthy donors, and intended for the treatment of B-ALL. It is genetically modified to avoid graft-versus-host disease (GvHD), and host-versus-graft rejection (HvG) while enhancing anti-tumor activity. RD06-03 can be prepared in a single batch for multiple people, achieving an "off-the-shelf" capability for patients in need of CAR-T cell therapy.
Related News
Contact Us







